A summary of findings from our post-doctoral fellow, Takao Tsukahara, as presented at the 10th SMS Research Symposium.
Takao Tsukahara, DDS, PhD, University of Michigan, Iwase and Sutton Lab
Knowledge gap.
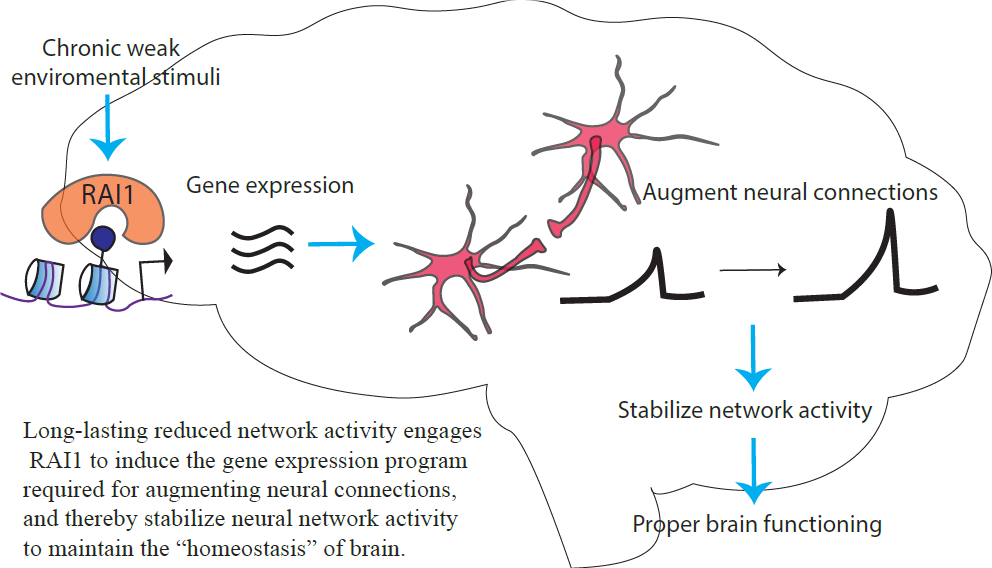
Smith-Magenis Syndrome (SMS) is a rare neurodevelopmental disorder characterized by intellectual disabilities, aberrant circadian rhythm and obesity. Previous studies have reported that Rai1, the causal gene of SMS, “reads” a chemical code written near the DNA and is involved in gene expression, especially at the transcription level. Transcription is the process in which DNA is converted into RNAs. RNAs in turn become proteins that build neurons and the brain. However, it remains unknown which genes RAI1 controls the transcription of in neurons.
One of the important roles of transcription is to change synaptic connectivity. The synapse is the functional connection between neurons. The flexible changes of synaptic connection in the brain in response to experience is called synaptic plasticity. Synaptic plasticity is an important basis of our learning behavior from experience in adults as well as for brain development of children. However, the role of Rai1 in synaptic plasticity is unknown.
Our research aims to fill these two knowledge gaps, thereby contributing to the understanding of the pathophysiology of SMS. Synaptic plasticity requires transcription induced by neuronal activity. Understanding the role of Rai1 in this process is important to mechanistically understand the pathology of SMS and develop therapeutic targets.
Is Rai1 involved in activity-dependent gene expression?
To address this question, we genetically reduced Rai1 in neural cultures and pharmacologically increased or decreased the neural network activity. Then, we chemically labeled the genes that were expressed in response to the neural network activity and compared them with genes expressed in cultures with normal levels of Rai1. Labeling only the newly expressed genes enabled us to profile these dynamic transcriptional events, which is not otherwise possible with conventional methods. Our genome-wide analysis revealed that loss of Rai1 specifically impairs the gene expression program driven by decreased network activity. This result indicates that Rai1 is required for gene expression program driven by decreased network activity.
Is Rai1 important for synaptic plasticity?
We addressed this question by carrying out a series of electrophysiological recordings, which allow us to measure the strength of the synapse. We found that the loss of Rai1 led to an inability to strengthen synaptic connections in response to reduced network activity. These results suggest that Rai1 controls transcription of genes in response to reduced network activity, which in turn strengthen synaptic connections. Our results raise an important possibility that, in SMS patients, the neural network is unable to fully respond to their daily experiences.
Furthermore, genes that are misregulated as a consequence of Rai1 reduction may represent novel therapeutic targets for SMS patients. We are now focusing on Per2, a crucial gene involved in the sleep-wake cycle, which is intensely misregulated in Rai1 reduced neuronal cultures. Interfering with Per2 may rescue the impaired homeostasis in neural network.